Abstract
*Deva Sharma, MD, MS, and Melissa Day, BS, contributed equally to the abstract and are co-first authors
Introduction:
Acute vaso-occlusive pain episodes are the primary reason for hospitalization in sickle cell disease (SCD) and predict mortality in adults. Women with SCD have higher pain rates than men, particularly during their reproductive years. Studies of the temporal relationship between the onset of menstruation and acute vaso-occlusive pain have reached conflicting conclusions. Healthcare providers often do not recognize the temporal relationship between the onset of menstruation and acute vaso-occlusive pain, with the common perception that acute vaso-occlusive pain is synonymous with dysmenorrhea. In sequentially independent cross-sectional studies involving discovery and confirmatory cohorts, we tested the hypothesis that acute vaso-occlusive pain is associated with the onset of menstruation in women with SCD and is distinct from dysmenorrhea. Evidence to support our hypothesis was based on identifying four distinct groups: 1) women with dysmenorrhea without acute vaso-occlusive pain associated with menstruation, 2) women with acute vaso-occlusive pain associated with menstruation without dysmenorrhea, 3) women with both, and 4) women with neither.
Methods:
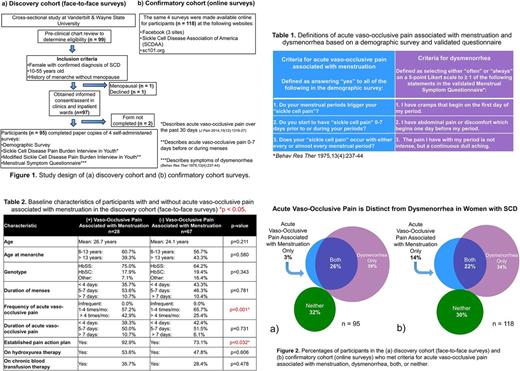
Discovery cohort : In an initial cross-sectional study, we interviewed women in the outpatient and inpatient settings at Vanderbilt University and Wayne State University (Figure 1). After obtaining informed consent, we conducted self-administered surveys which included a validated questionnaire to define acute vaso-occlusive pain associated with menstruation and dysmenorrhea (Behav Res Ther 1975, 13(4):237-44). Acute vaso-occlusive pain associated with menstruation and dysmenorrhea were defined by temporal and frequency criteria so that participants could meet either or both criteria (Table 1). Associations of clinical and demographic variables with acute vaso-occlusive pain triggered by menstruation and dysmenorrhea were evaluated by the chi-square test and t-test, and then in a multivariable regression model. The proportions of women who met criteria for acute vaso-occlusive pain associated with menstruation and dysmenorrhea were determined. A p-value of 0.05 was considered significant for all analyses.
Confirmatory cohort : In a subsequent cross-sectional study, we conducted an identical online questionnaire using select websites (Figure 1) to validate our initial findings. Statistical analyses were identical to the discovery cohort.
Results:
Discovery cohort : A total of 95 women were surveyed using face-to-face questionnaires. Baseline characteristics are summarized in Table 2. Twenty-eight women (29.5%) met defined criteria for acute vaso-occlusive pain associated with menstruation. Age (p=0.211) and genotype (p=0.343) were not associated with acute vaso-occlusive pain triggered by menstruation. Sixty-two women (65.3%) met criteria for dysmenorrhea. Importantly, among the 95 participants in the discovery cohort, 3% met criteria for acute vaso-occlusive pain associated with menstruation alone, in the absence of dysmenorrhea, while 26% met criteria for both acute vaso-occlusive pain associated with menstruation and dysmenorrhea (Figure 2).
Confirmatory cohort : A total of 118 women were surveyed using the online questionnaires. Baseline characteristics were comparable between the discovery and confirmatory cohorts. Forty-three women (36.4%) met defined criteria for acute vaso-occlusive pain associated with menstruation. Sixty-six women (55.9%) met criteria for dysmenorrhea. Importantly, among the 118 participants in the confirmatory cohort, 14% met criteria for acute vaso-occlusive pain associated with menstruation alone, in the absence of dysmenorrhea, while 22% met criteria for both acute vaso-occlusive pain associated with menstruation and dysmenorrhea (Figure 2).
Conclusions:
The onset of menstruation in women with SCD is another, yet unrecognized risk factor for acute vaso-occlusive pain episodes. Further studies are needed to better define the underlying mechanism and facilitate the development of targeted therapies for affected women.
Rodeghier: Rodeghier consultants: Consultancy. Callaghan: Pfizer Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Site PI, Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Site PI, Speakers Bureau; Roche; Shire: Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Grifols: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Speakers Bureau; Alnylam Pharmaceuticals, Inc: Other: Owns stock, stock options, or bonds ; Global Blood Therapeutics: Other: Site PI; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Biogen: Membership on an entity's Board of Directors or advisory committees; Sancillio: Other: Site PI; Bayer HealthCare; Pfizer Inc.; Roche; Shire: Consultancy; Baxalta: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.